Cancer Treatment Goes Viral

Image: Centers for Disease Control and Prevention / Dr. G. William Gary Jr.
Editor’s Note: This story originally appeared in the winter 2023 issue of NEXT magazine. Our online version includes more stories about innovative research happening on the MCV Campus.
By Paul Brockwell Jr.
Cancer cells are often hard to target effectively. By their nature, the unchecked growth of tumors sends them silently spreading throughout the body, replicating and continuing to change and evolve genetically. Tumor cells, if they are not already resistant to traditional treatments like chemotherapy and radiation, may be growing in areas of the body where surgical intervention is too risky or impossible. Those pernicious challenges have inspired VCU researchers to create a treatment that can specifically target and destroy cancer cells without harming healthy cells.
Similar approaches have used the body’s own immune cells to target cancer, but in this scenario, imagine a deadly agent dispatched — like a heat-seeking missile — to selectively take out problem cells that have transformed into cancerous growth engines. Once the initial scuffle of a viral takedown begins, the byproducts of an immune response — the cytokines the body secretes to coordinate further assistance — stay on the scene, stamping out surrounding cancerous cells and securing the perimeter.
The researchers started by looking at the limiting factors preventing the body’s normal immune processes from working better, and they eventually identified research that could translate to a clinical setting by focusing on how to perfect cancer-targeting viruses using a novel delivery method.

That imagined scenario may be a reality soon, thanks to several researchers at the VCU Institute of Molecular Medicine (VIMM) led by Paul B. Fisher, M.P.H., Ph.D., FNAI, professor and chair of the Department of Human and Molecular Genetics in the VCU School of Medicine and the Thelma Newmeyer Corman Chair in Cancer Research at VCU Massey Cancer Center. Dr. Fisher founded the VIMM at the VCU School of Medicine and has served as its director since 2008. He also co-founded the Cancer Molecular Genetics research program at Massey and co-led it as program leader for nine years.
“I’m really excited about discovering and understanding how molecules control complex biological processes,” Dr. Fisher said. “Seeing our novel molecules translate into lifesaving cancer treatment is a major accomplishment and the most rewarding part of my work.”
Dr. Fisher’s team has engineered adenoviruses that contain parts of two different adenoviruses, serotype 5 and serotype 3 (Ad.5/3), to selectively target cancer cells. Normally, adenoviruses are known for causing the common cold in humans, but their modified viral agent (Ad.5/3) has an enhanced ability to infect cancer cells where replication of the virus is controlled by a gene region called a promoter that is cancer-selective in its expression. In normal cells, this promoter gene is inactive and will not send the virus’s replication into hyperdrive. In cancer cells, the promoter gene is switched on and attacks the tumor cells by replicating with abandon, bursting out of the cell and seeking out additional cellular victims nearby and at a distance.
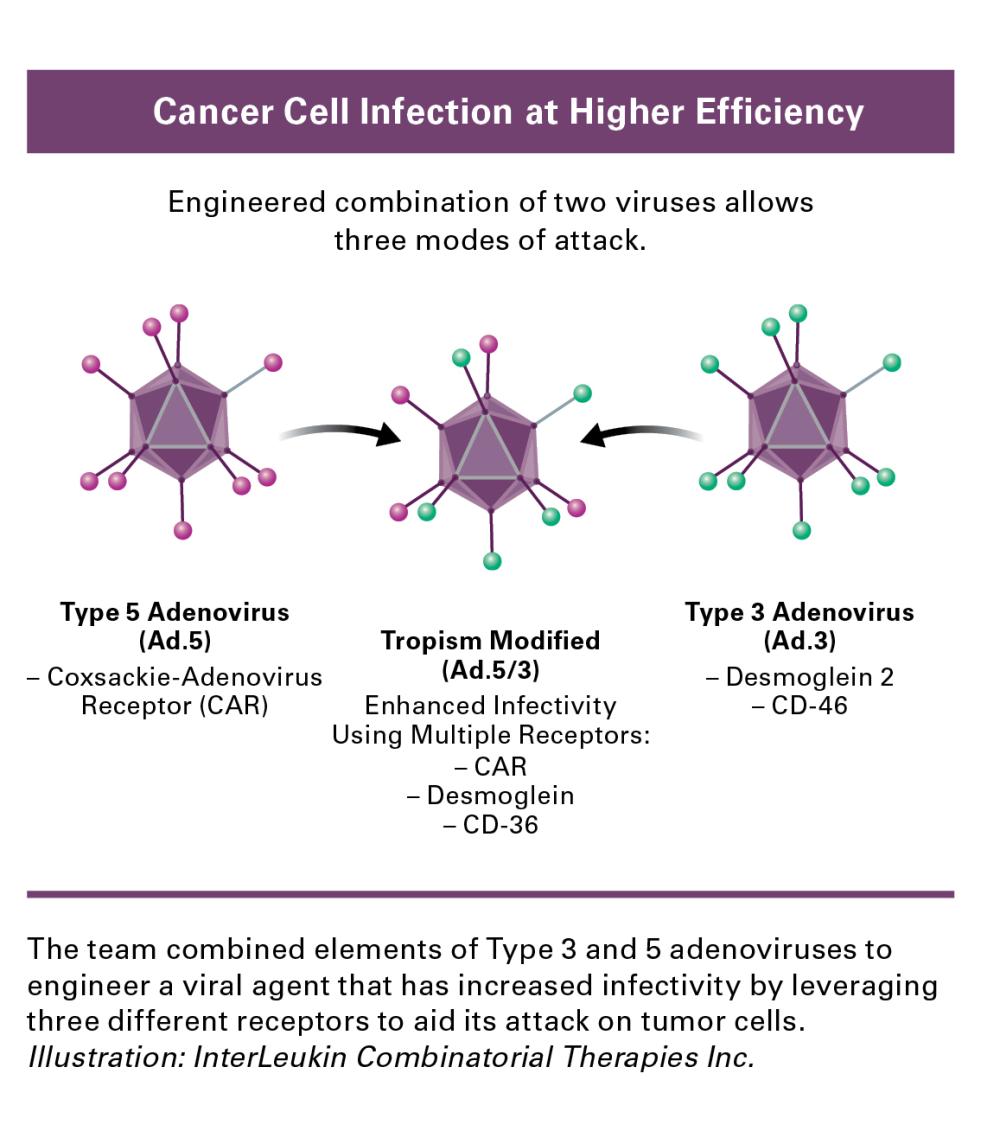
To enhance cancer-selective therapy with Ad.5/3, Dr. Fisher’s team created a two-part cancer terminator virus (CTV). In addition to cancer-specific virus replication the CTV selectively expresses a gene producing a secreted protein, the cytokine interleukin 24 (IL-24), which can uniquely kill cancer cells locally and at a distance, inhibit new tumor blood vessel formation, make chemotherapy and immune therapy work better, and stimulate the immune system to produce a memory effect that prevents tumor regrowth. This treatment method with a novel CTV delivers a one-two punch: First, the virus attacks, replicates and causes cell death in tumorous cells. A second promoter gene selectively drives production of IL-24 when viral replication is induced. Like calling for backup support, this cytokine deploys additional immune responses to tumor cells nearby while avoiding healthy cells and attacks cancer cells at a distance, including tumor cells that have spread (metastasized) to other sites in the body.
The biggest benefit of this approach, said Dr. Fisher, is that the CTV is genetically agnostic. Cancer is notorious for shape-shifting, evolving and mutating in ways that allow it to outwit current treatments, but those tactics would not allow it to escape or out-morph the cancer-targeting viral agent and its trusty cytokine sidekick, IL-24.
The researchers started by looking at the limiting factors preventing the body’s normal immune processes from working better, and eventually identified research that could translate to a clinical setting by focusing on how to perfect cancer-targeting viruses using a novel delivery method.
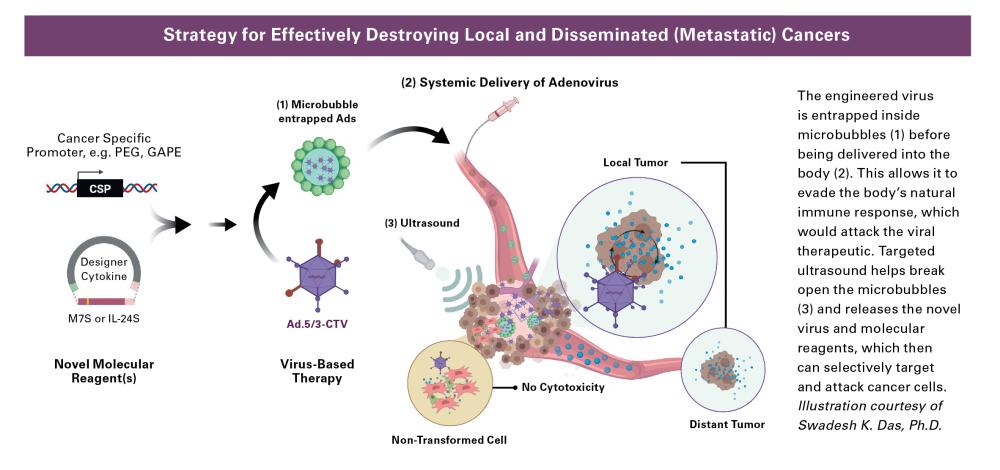
The biggest challenges for this approach when delivering cancer-targeting viruses into circulation has been figuring out how to sneak past the body’s immune system and prevent nonspecific trapping in the liver. Dr. Fisher and his team also needed to find a way to deliver these viral hunters without triggering the alarms on the body’s internal immune system.
 Slipping past security requires a new take on the Trojan horse. To do that, the cancer-targeting viral particles (CTVs) are encased in microbubbles that protect the virus from being identified and targeted by the body’s natural immune response before it can attack the tumor cells. When the injection is complete, targeted ultrasound vibrations burst the microbubbles open and release the virus to do its work.
Slipping past security requires a new take on the Trojan horse. To do that, the cancer-targeting viral particles (CTVs) are encased in microbubbles that protect the virus from being identified and targeted by the body’s natural immune response before it can attack the tumor cells. When the injection is complete, targeted ultrasound vibrations burst the microbubbles open and release the virus to do its work.
As discussed above, the researchers have also engineered IL-24 — an immune system byproduct — into the virus (Ad.5/3-CTV) to tackle uninfected cells in the surrounding area and at a distance. IL-24’s secretion generates additional cancer-killing properties and goes off like a signal flare to dispatch other immune cells to target nearby cancer cells. While the release of infectious virus by the initially infected tumors cells is restricted over time by the immune system, IL-24 can circulate through the body, induce its own synthesis and selectively kill tumor cells directly and through enhancement of the body’s immune responses.
Dr. Fisher and his team hope that cancer-targeting viruses and the designer cytokine treatments will have a diversity of applications across specific cancer types where traditional treatments might fail.
In 2020, Dr. Fisher and Devanand Sarkar, Ph.D., worked with VCU Innovation Gateway to patent a new class of cancer-targeting adenoviruses (Ad.5/3-CTV) they worked on together. A crucial part of the process, Dr. Fisher said, was securing the intellectual property protections for the team’s work on the adenovirus with a patent along with the system for delivering the virus and its designer cytokine byproduct left to clean house on tumor cells. Ad.5/3-CTV, ILCT-5307 has been exclusively licensed to a biotechnology startup company, InterLeukin Combinatorial Therapies Inc., to bring this novel therapeutic into the clinic to treat patients with recurrent glioblastoma (GBM), an invariably fatal cancer without any effective therapy.
What’s Next?
Dr. Fisher and his team hope that cancer-targeting viruses and the designer cytokine treatments will have a diversity of applications across specific cancer types where traditional treatments might fail. They have published on melanoma, breast cancer, prostate cancer, neuroblastoma, colorectal carcinoma, ovarian cancer and glioblastoma. To date, they have not found a cancer resistant to the promoter gene being expressed to target viruses to replicate in tumor cells.
Last year, they began preclinical studies using a contract research organization to evaluate any potential toxicity of Ad.5/3-CTV (ILCT-5307) when injected in the brains of animal models. No toxicity or behavioral changes were observed and the same protocol will initially be used to treat human patients with glioblastomas. In preclinical animal models, a single injection of the virus cured 50% of animals. These strong results are the culmination of years of work in the lab.
“Science is an iterative, progressive process,” Dr. Fisher said. “It takes minutes or seconds to identify a gene that may be important, but it can take a lifetime to understand how that gene works.”
It’s a pivotal moment, Dr. Fisher said, when years of basic science research are on the cusp of being translated into clinical applications. He also shared how important colleagues like Dr. Sarkar; Shawn Wang, Ph.D.; Luni Emdad, Ph.D.; and Swadesh K. Das, Ph.D., have been to the progress. Dr. Wang co-leads the Developmental Therapeutics Group at Massey and is associate director of immunology in VIMM. Drs. Emdad and Das are associate professors in human and molecular genetics and affiliated with Massey and the Institute for Molecular Medicine. Their research collaboration has moved the project forward on the MCV Campus and positioned the research to be an early success story of the Molecules to Medicine Program at VCU Massey Cancer Center.
We’re extremely excited and optimistic about where this research is going.
Paul B. Fisher, M.P.H., Ph.D., FNAI
The project received critical early funding from the National Foundation for Cancer Research for work in the lab, and private philanthropy helped finance the preclinical studies. The team is now raising money to complete the clinical trials, and they’ve received interest from multiple institutions across the U.S. Glioblastomas, one of their early targets, are an aggressive form of brain cancer that is relatively rare compared to other cancers in the body. That reality makes partnering with other institutions like M.D. Anderson, Johns Hopkins and Northwestern necessary to recruit adequate numbers of patients with recurrent glioblastomas. The Ad.5/3-CTV is also effective against brain tumors that originally develop outside the brain and then metastasize, which account for 30% to 40% of brain tumors. This potentially expands the scope and utility of therapeutic viruses in patients with both primary brain tumors (GBM) or secondary brain tumors (a consequence of metastasis from organs outside the brain).
From here, the team hopes to enter clinical trials with its cancer-targeting viruses, but its long-term vision includes examining and developing other candidates for designer viruses and designer cytokines that can help tackle liver and pancreatic cancers, as well as other hard-to-treat or resistant tumors by leveraging the viruses that can selectively strike cancer cells. Team members also could see the potential for genetically modifying other immune cells in patients to attack cancers and work in concert with other therapies to drive better patient outcomes.
“We’re extremely excited and optimistic about where this research is going,” Dr. Fisher said. “This could be a major game changer and a paradigm shift in how aggressive and metastatic cancers are treated clinically.”
If you are interested in supporting this research effort at VCU Massey Cancer Center, please contact Jasmine J. Davis, senior director of development with the Office of Medical Philanthropy and Alumni Relations, at 804-484-4903 or jjdavis3@vcu.edu.
Make a Difference
Support cancer research at VCU Massey Cancer Center for a better future.