Turning Off the Engines of Metastatic Growth

By Paul Brockwell Jr.
Metastasis starts with a cancer cell’s escape from a primary tumor and into the bloodstream. Over time, tumor cells land in other locations and form a cluster of new cells. Dysregulated cells continue to grow and wreak additional havoc by branching out, invading organs and tissues throughout the body.
The spread of cancer through metastases accounts for an estimated 90% of cancer deaths in patients with solid cancers, a grim statistic that has changed very little over the past 50 years.1 The process almost always worsens a patient’s prognosis because once cancer has embedded throughout the body, detection and treatment become inefficient and ineffective.
What’s most exciting is that our preclinical studies confirm that inhibiting MDA-9 either genetically or pharmacologically can profoundly suppress metastasis.
Swadesh K. Das, Ph.D., associate professor, VCU School of Medicine
VCU researchers are working to understand the complicated protein interactions that drive tumor cell growth, and they’ve invented a molecule that shows great potential for stopping metastatic growth in its tracks. That goal has been the focus of research teams at the VCU Institute of Molecular Medicine (VIMM) and VCU Massey Comprehensive Cancer Center, which are developing novel ways of targeting the processes that fuel such unregulated cell growth and stopping the expansion of cancer cells before they take root in other parts of the body.
“Our interest and focus over the years have been to comprehend and define ways to attack metastases,” said Paul B. Fisher, M.Ph., Ph.D., professor and previous chair in the Department of Human and Molecular Genetics at the VCU School of Medicine. “Achieving success in this quest has potential to directly translate into therapies for multiple types of advanced aggressive cancers.”
Dr. Fisher holds the Thelma Newmeyer Corman Chair in Cancer Research at Massey. He founded the VIMM at the School of Medicine and has served as its director since 2008. He co-founded the Cancer Molecular Genetics research program at Massey and helped lead it for nine years. In 2017, Dr. Fisher was named a fellow of the National Academy of Inventors.
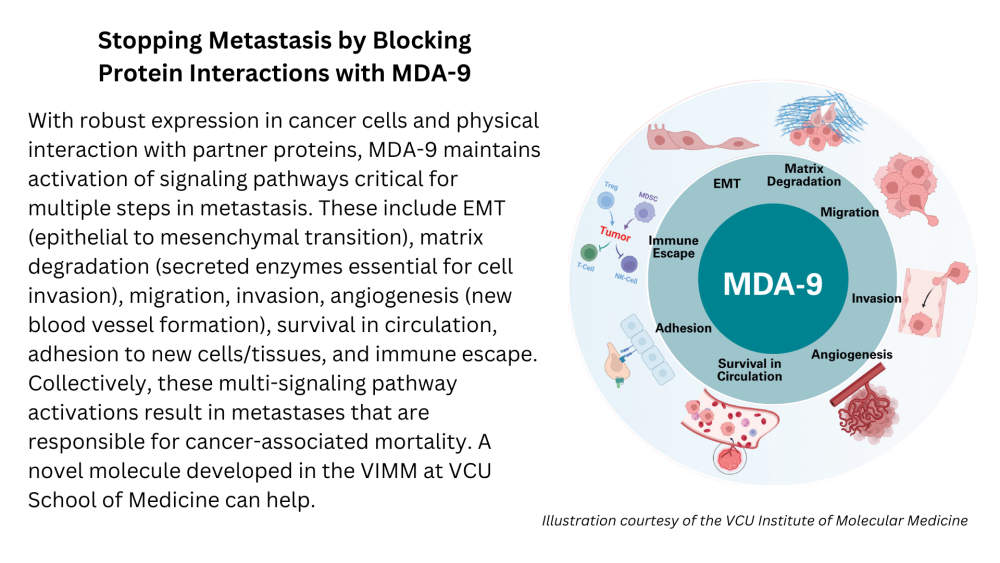
Along with Swadesh K. Das, Ph.D., an associate professor and key VIMM member, Dr. Fisher and the team have been exploring ways to interrupt the processes that drive metastatic tumor growth through inhibiting a specific pro-metastatic gene called melanoma differentiation associated gene-9, or MDA-9. When active, this protein plays a key role in driving metastatic growth.
“An intriguing and important property of MDA-9 is its ability to regulate many of the critical steps of metastasis in an expansive array of metastases originating from different organ sites in the body,” Dr. Fisher said. “This property suggests that inhibiting the activity of this protein could in principle have broad applications in the therapy of multiple metastasizing cancers.”
Drs. Fisher and Das noticed additional benefits to blocking MDA-9 in metastatic cells — doing so makes tumor cells more sensitive to a second therapeutic agent and makes the cellular environment more hostile to cancer growth.
“What’s most exciting is that our preclinical studies confirm that inhibiting MDA-9 either genetically or pharmacologically can profoundly suppress metastasis,” Dr. Das said. “Unraveling the mechanisms of this complicated process continues, but our ability to effectively target and treat metastasis to decrease patient morbidity and mortality has found a potentially powerful tool when targeting and inhibiting this gene.”
Additionally, they were able to show that the MDA-9 gene regulates key components of the immune system and blocking MDA-9 increases sensitivity of cancers to immune destruction. This key research was done in collaboration with fellow faculty in the Department of Human and Molecular Genetics and VIMM: Xiang-Yang (Shawn) Wang, Ph.D., professor, associate director of immunology at VIMM and co-leader of Massey’s Developmental Therapeutics Program, and Devanand Sarkar, M.B.B.S., Ph.D., professor, associate director of cancer therapeutics at the VIMM and Massey’s associate director for cancer research training and education coordination.
ISOLATING A KEY METASTASIS PROTEIN
Dr. Fisher’s team needed to find ways to effectively disrupt the cellular processes driving metastasis. They examined the genetic information produced in growing metastatic melanoma cells vs. metastatic melanoma cells chemically induced to stop growing and revert to a noncancerous state, a form of cancer differentiation therapy. A molecular approach called subtraction hybridization identified the MDA-9 protein as an upregulated molecule associated with melanoma metastasis. By examining the genes and proteins mapped in the Human Genome Project and through direct laboratory and preclinical animal studies, the team established that MDA-9 is a critical protein in cancer progression and can affect a broad spectrum of different cancers.
“We proposed two simple hypotheses: first, could we identify the genes and proteins that might be critical in regulating various stages in the metastatic process?” Dr. Fisher said. “Most approaches have focused on one or at most a few of the key steps. However, in many instances, tumor cells may develop compensatory mechanisms avoiding single-agent therapies. By blocking multiple stages simultaneously, the tumor cells may fail to overcome inhibition. Accordingly, our second hypothesis was that we should look for proteins that regulate the metastatic process at multiple steps, thinking that if we simultaneously inhibit several key regulatory pathways, we could cut off multiple avenues controlling tumor cell progression to metastasis.”
Metastasis is a multistep process involving several critical stages. First, a cancer cell must leave the primary tumor (intravasation), survive in a patient’s bloodstream and begin to invade new tissues or organs (extravasation) before it grows and builds the cellular infrastructure to support the new tumor cell growth. An essential component of continued tumor growth and expansion is angiogenesis, or the development of new blood vessels.

A key to why targeting MDA-9 might be a more feasible approach to block metastasis than trying to block only one or a few of the stages comes from research indicating that MDA-9 is active in most, if not all, of the major stages of metastasis. Since one of its major functions is invasion, MDA-9 was considered a prime target for therapeutic interventions.
BLOCKING MDA-9 PROTEIN INTERACTIONS INHIBITS METASTASIS
The MDA-9 protein contains two similar PDZ domains, PDZ1 and PDZ2. PDZ domains are commonly found amino acid structures that play a key role as signaling proteins. These domains are essentially the temporary staging areas for organizing and maintaining complicated protein interactions that are needed to initiate transcription of the genetic code required for both normal and abnormal cell physiology. In a cell, they are construction sites for new growth and transformed properties of a cell. For a cancer cell, the problem is that these signaling domains are working from corrupted blueprints. Interrupting their functionality helps shut down the work site and inhibit cancer-associated and metastasis-associated processes.
The exciting part of identifying a potential small molecule inhibitor is that you can directly evaluate its biological and pharmacological properties to determine the possibility that this molecule might be progressed as a therapeutic agent.
Paul B. Fisher, M.Ph., Ph.D., professor and previous chair in the Department of Human and Molecular Genetics at the VCU School of Medicine
Since PDZ domains with similar sequences are found in a vast number of proteins, it was considered unlikely that specific inhibitors of the MDA-9 PDZ domains could be identified. Put more simply, this protein was considered “undruggable.” In collaboration, Drs. Fisher and Maurizio Pellecchia, professor of medicinal chemistry at the Sanford Burnham Prebys Medical Discovery Institute, La Jolla, Calif., where Dr. Fisher is a visiting professor, proved the protein was, in fact, druggable.
They employed a novel strategy, called fragment-based drug discovery (FBDD) informed by nuclear magnetic resonance (NMR), to identify small molecules that could bind specifically to the PDZ domains of MDA-9, but not similar PDZ domains in other proteins. This work resulted in the identification of a first-in-class inhibitor of the MDA-9 PDZ1 domain, called PDZ1i (IVMT-Rx-1).2
When initially tested in brain tumor models, PDZ1i inhibited invasion of glioblastoma, prolonging survival, which was enhanced even further when combined with the standard-of-care radiation. It is worth noting the inhibitor identified by Dr. Fisher was included in studies published in Proceedings of the National Academy of Sciences and highlighted in the journal Science as a potential future drug for brain cancer treatment.
“The exciting part of identifying a potential small molecule inhibitor is that you can directly evaluate its biological and pharmacological properties to determine the possibility that this molecule might be progressed as a therapeutic agent,” Dr. Fisher said. “After examining these aspects, our first-in-class PDZ inhibitor looked promising. The molecule was bioactive, nontoxic and had a long half-life in serum; it did not disappear after eight hours. Unfortunately, it was poorly soluble, suggesting that it could not be formulated without further modification to be used in a patient.”
In preclinical animal modeling, PDZ1i was shown to be very active in inhibiting invasion and metastasis in a broad spectrum of cancers, including brain (glioblastoma), neuroblastoma, prostate, breast, liver and pancreas. The team’s initial PDZ1 inhibitor looked promising and provided a proof of principle for drugging a specific MDA-9 PDZ domain; however, they needed to make it more soluble in order to be able to deliver the molecules where needed and to allow for detailed toxicology testing prior to human use.
Luckily, they had been introduced to a wet milling technique where ground up a large molecule with zirconium beads to make nano crystals with the same properties as the original inhibitor. The result was smaller and soluble molecules that could be used in an intravenous solution for human patients, with the hope of achieving the same results observed in preclinical animal models.
CREATING A DUAL INHIBITOR
Previous studies in the Fisher and Das laboratories suggested that both PDZ domains are necessary in defining the many attributes of MDA-9’s role in metastatic cell growth. To address this challenge, the team created a novel hybrid molecule called IVMT-Rx-3 that binds to both domains and effectively blocks interactions with relevant cancer partner proteins.
“In principle, we hypothesized that this molecule might prove more active than a single PDZ domain inhibitor in maximizing disruption of MDA-9 protein signaling and many of MDA-9’s pro-metastatic activities,” Dr. Fisher said.
To test their solution and prediction, the team picked the gnarliest and most aggressive metastatic melanoma models that do not respond to the most advanced immunotherapies.
IVMT-Rx-3 has good pharmacological properties, does not show toxicity toward normal pigmented skin cells, blocks invasion of metastatic melanoma cells and is well tolerated and clinically effective in a preclinical animal model. An added advantage of IVMT-Rx-3 is its solubility in solutions that could potentially allow formulation for use in patients. By itself, IVMT-Rx-3 inhibited development of lung metastases by the aggressive and immune therapy-resistant B16 metastatic melanoma cells.

To further enhance therapeutic outcomes, IVMT-Rx-3 was combined with an immune checkpoint inhibitor, a type of immunotherapy that helps maximize the host’s natural immune system activity by preventing, blunting or negating the immunological impact of the novel therapeutic molecules. This solution worked, allowing immunotherapy to be effective and eliminating metastatic growth in animal models with these more soluble molecules, resulting in repression of melanoma metastasis. But the benefits did not end there.
“In new follow-up studies, we noticed that blocking the MDA-9 gene or protein inhibits the ability to transport chemotherapy out of cancer cells,” Drs. Fisher and Das said. “Accordingly, physicians may be able to use lower chemotherapy doses and combine them with MDA-9 PDZ inhibitor treatment, which retains the therapy in the tumor cell. These molecules synergize with existing standards of care and can effectively block metastatic growth by causing tumor cell death.”
What’s Next?
Dr. Fisher and his team hope to move this MDA-9 inhibitor treatment through toxicology studies to show that the biochemical properties are not harmful to patients and can be adjusted and translated into a clinical model.

The road to the clinic will ultimately involve detailed toxicology to define the safety of the small molecule and to identify combination strategies that can be translated into effective therapies for patients with advanced and aggressive metastatic and invasive cancers.
“We hope to advance specific MDA-9 PDZ inhibitors from bench to bedside to prevent and treat patients with metastatic cancers. The biggest gap in advancing small molecules is always doing this last part,” Dr. Fisher said. “We’re at a point where we do not think our treatments are toxic since they kill cancer cells indirectly through the immune system and by inhibiting other components of metastasis, but they need to undergo rigorous testing to ensure they’re safe before early-stage clinical safety and future therapeutic trials can be performed with MDA-9 PDZ inhibitors in human subjects.”
Additional limiting factors in advancing promising technologies into the clinic involve the costs associated with detailed toxicology, making patient-ready small molecules and performing the actual clinical trials. For the latter challenge, the team continues to work with VCU’s Molecules to Medicine program, the VIMM and a biotechnology company, InVaMet Therapeutics Inc. (IVMT), to create and develop the molecules needed to continue their research. To accelerate the MDA-9 PDZ inhibitor program from bench to bedside, Drs. Fisher and Webster K. Cavenee formed IVMT with funding from private philanthropy and the National Foundation for Cancer Research. The current PDZ inhibitors, including IVMT-Rx-1 and IVMT-Rx-3, are exclusively licensed to IVMT to expedite translation of these small molecules from the laboratory into the clinic to treat patients with aggressive advanced cancers.
Their work with the new chimeric inhibitor IVMT-Rx-3 is gaining the notice of researchers around the world. Molecular Cancer Therapeutics, an American Association for Cancer Research journal of cancer drug discovery and preclinical research, highlighted this new compound as a “First Disclosure” of a novel therapeutic agent and also featured the lab’s model for IVMT-Rx-3 mode of action on the cover of its October 2023 issue.3
“In principle, our new results support the feasibility of engineering MDA-9, dual-PDZ inhibitors with enhanced antimetastatic activities and applications of our novel molecule to a broader array of human cancer metastasis caused by an overexpression of this particular pro-metastatic gene,” Dr. Fisher said.
Such a treatment could turn off the engines of metastasis and inhibit the ability of tumor cells to grow and spread throughout the body. With more research, Drs. Fisher and Das hope their teams may be able to test a theoretical universal inhibitor for the key steps of metastasis that would complement other standards of care to ensure better patient outcomes.
“We believe our small molecule inhibitors could be incorporated to help treat many different types of cancers,” Dr. Fisher said. “Combined with other available standard-of-care and new innovative therapies, we hope to provide powerful tools for stopping the spread of cancer as patients receive other treatments and a strategy that also attacks resident metastatic tumor cells.”
If you are interested in supporting this research effort at VCU Massey Comprehensive Cancer Center, please contact Jasmine J. Davis, senior director of development in the Office of Medical Philanthropy and Alumni Relations, at 804-484-4903 or jjdavis3@vcu.edu.
1. Chaffer, C.L., Weinberg, R.A. A perspective on cancer cell metastasis. Science. 2011;331(6024):1,559–64.
2. Kegelman, T.P., Wu, B., Das, S.K., Talukdar, S., Beckta, J.M., Hu, B., Emdad, L., Valerie, K., Sarkar, D., Furnari, F.B., Cavenee, W.K., Wei, J., Purves, A., De, S.K., Pellecchia, M., Fisher, P.B. Inhibition of radiation-induced glioblastoma invasion by genetic and pharmacological targeting of MDA-9/Syntenin. Proceedings of the National Academy of Sciences. 2017;114(2):370–75.
3. Pradhan, A.K., Modi, J., Maji, S., Kumar, A., Bhoopathi, P., Mannangatti, P., Guo, C., Afosah, D.K., Mochel, M.C., Mukhopadhyay, N.D., Kirkwood, J.M., Wang, X.Y., Desai, U.R., Sarkar, D., Emdad, L., Das, S.K., Fisher, P.B. Dual Targeting of the PDZ1 and PDZ2 Domains of MDA-9/Syntenin Inhibits Melanoma Metastasis. Molecular Cancer Therapeutics. 2023;22(10):1115–27.
Discover More
Read more features like this in the winter issue of NEXT magazine.